Renerva Begins First-in-Human Study for Novel Device to Prevent Neuroma Pain
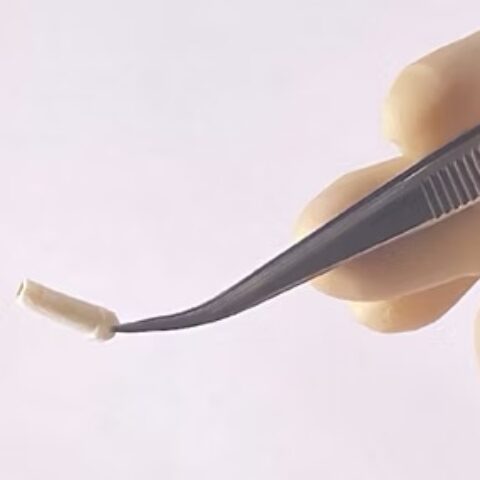
Renerva, Inc., a Center for Life Science Ventures client developing medical devices for peripheral nerve repair, has received approval from the U.S. Food and Drug Administration to launch a first-in-human clinical study of its Renerva PNM-CAP™ nerve-cap device.
The study will take place at The Ohio State University Wexner Medical Center and will be led by Principal Investigator Dr. Amy M. Moore, MD, who holds the Robert L. Ruberg MD Alumni Endowed Chair of the Department of Plastic and Reconstructive Surgery .
The device is designed to inhibit neuroma, which is disorganized nerve growth that occurs after a nerve is severed. Neuromas often develop after extremity amputations or other nerve injuries, as the ends of a nerve attempt to regenerate but can’t find a target. The resulting formation of a tangled mass of nerve fibers causes chronic, intractable pain for many of the nation’s two million amputees, often leading to reliance on opioids and reduced quality of life.
“Receiving IDE approval is a pivotal milestone for Renerva, transitioning us into a clinical-stage company and significantly de-risking our technology path,” said Lorenzo Soletti, CEO of Renerva. “This achievement, which allows us to proceed with our first-in-human study, is a major validation of our science and a critical value inflection point for the company. We are honored to partner with Dr. Amy M. Moore and her world-class team at The Ohio State University, whose unparalleled expertise will be invaluable as we work to establish a new standard of care.”
Implanted at the end of a transected nerve, the Renerva PNM-CAP™ device curtails neuroma development by providing small, separated paths for nerve fibers, or axons, to penetrate as they attempt to regrow. By guiding and limiting axonal growth, the device allows axons to gradually exhaust their regenerative potential while remaining isolated from one another, making neuroma formation less likely. The device also shields the nerve ending from surrounding tissue signals that can trigger disorganized growth.
The IDE approval for the device was supported by robust preclinical data published recently in npj Regenerative Medicine. In those studies, the Renerva PNM-CAP™ device reduced nerve growth by as much as 16-fold compared with standard care and demonstrated a 3.5-fold reduction in average pain behavior over four months.
“Our preclinical studies demonstrate that the PNM-CAP technology has a profound impact on inhibiting nerve growth, a key event in neuroma formation,” said Bryan Brown, Chief Technology Officer of Renerva.
The upcoming clinical trial is designed to provide the first evidence of how the Renerva PNM-CAP™ device performs in patients.
Founded in 2017 at Cornell and the University of Pittsburgh, Renerva has incubated with the CLSV since 2020. The company is headquartered in Pittsburgh with a satellite location in Ithaca.
